Fungi associated with water from gallons of truck pushers sold within Sokoto metropolis and their susceptibility profile to different antifungal drugs
Abstract
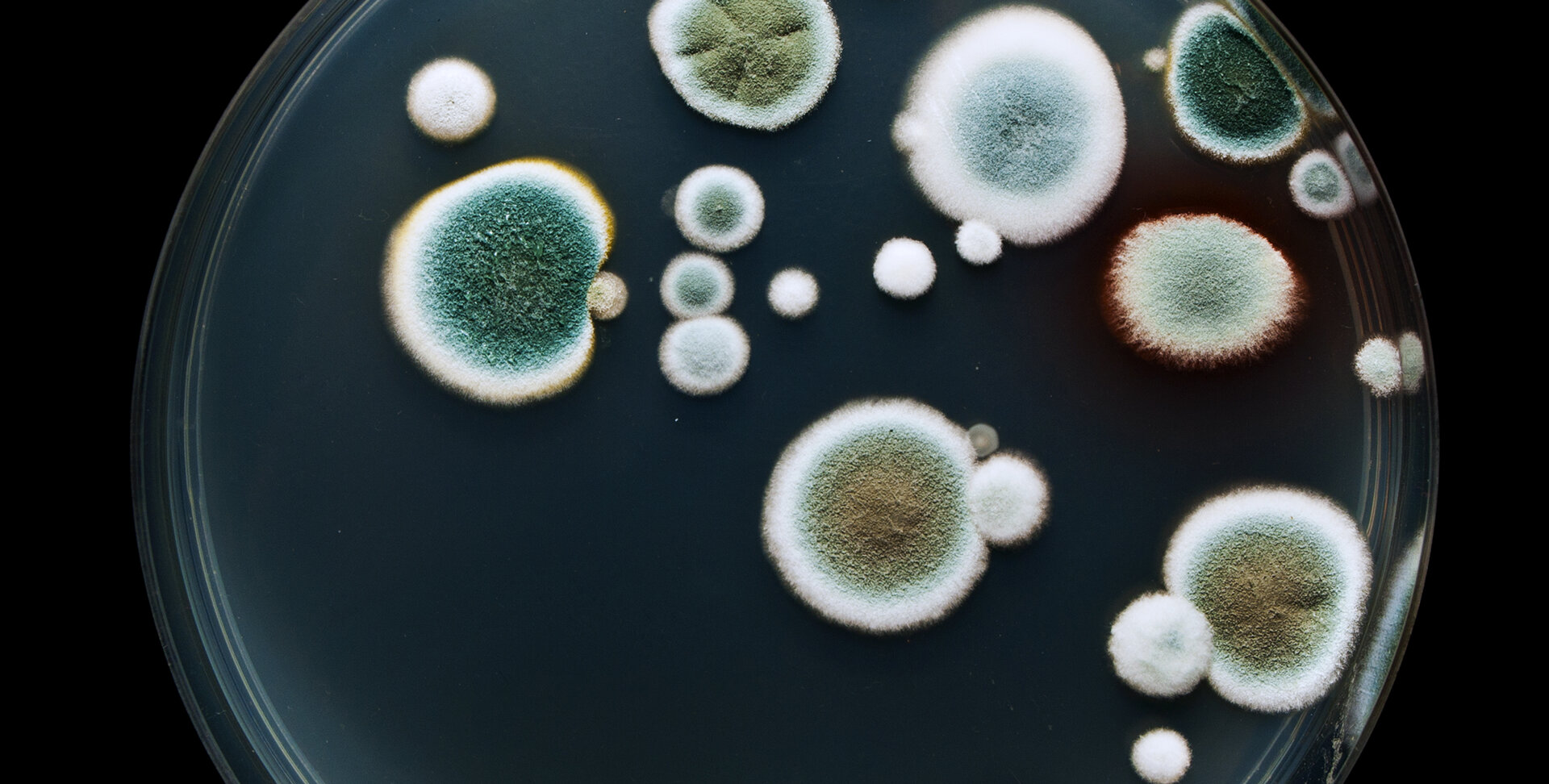
This research was conducted to isolate fungi from water in gallons of truck pushers and determine their susceptibility to different antifungal drugs. The water samples from different areas in Sokoto were collected by the use of sterile plastic container and analyzed. During this research, serial dilution, isolation and identification were done. From the result obtained, different colonies were observed after incubation period for 7-14 days. The highest mean colony count was 2.4x103cfu/ml and the least mean colony count was 1.6x103cfu/ml. The fungi isolated include Aspergillus niger, Apergillus flavus, Aspergillus fumigatus, Aspergillus oryzae and Rhizopus stolonifer with Apergillus niger having the highest percentage frequency of occurrence (34%) while Rhizopus stolonifer had the least percentage frequency of occurrence (4%). Each of the fungal isolates was tested against the following antifungals: Itraconazole, ketoconazole, Griseofulvin and Fluconazole each at a concentration of l0mg/ml, 40mg/ml and 60mg/ml. In griseofulvin, a 100% zone of inhibition was recorded against Aspergillus flavus at the concentration of 60mg/ml while in Aspergillus niger it was 75%, and in Aspergillus fumigatus it was 87.5%, Aspergillus oryzae 81.25% and Rhizopus stolonifer 90% at concentration of 60mg/ml. In Itraconazole, the fungal isolates were more susceptible at concentration of 60mg/ml in which Aspergillus flavus showed 100% zone of inhibition while Aspergillus niger showed 81.75%, Aspergillus fumigatus was 93.75%, Aspergillus oryzae was 81.25% and Rhizopus stolonifer was 91.25% at concentration of 60mg/ml. Also in ketoconazole, the isolate Rhizopus stolonifer and Aspergillus fumigatus showed 100% zone of inhibition at different concentrations of 10mg/ml, 40mg/ml and 60mg/ml. while Aspergillus niger was recorded at 87.50%, Aspergillus flavus was 93.75% and Aspergillus oryzae was 91.25% at concentration of 60mg/ml. fluconazole was the most effective against the fungal isolates as 100% zone of inhibition was recorded at concentration of 60mg/ml for all of the fungal isolates as well as Aspergillus fumigatus at concentration of 40mg/ml was recorded as 100% zone of inhibition among the tested antifungals for susceptibility testing. However, the zone of inhibition measured showed that the antifungals at all given concentrations were active against the fungal isolates. Conclusively, fungi are present in water sample and they include both pathogenic and saprophytic species. Thus attention should be given to water treatment quality and gallons used by truck pushers should always be clean to improve in hygienic practices.
References
Adriano, A.B., Joana, S.B., 2007. The quest for safe drinking water: An example from Guinea-Bissau (West Africa). Water res. 41 2978-2986. Akunyili, D., 2005. Good potable water in the society. NAFDAC information bulleting, 4, 5-6.
Ana, B.G., Russel, R.M., Nelson, L., 2006. Survey and significance of fungi in tap water. Int. J. Hyg. Environ. Health, 209, 257-264.
Annaise, E.J., Stratton, S.L., Dignani, M.C., Summerbell, R.C., Rex, J.H., Monson, T.P., Spencer, T., Kasai, M., Francesconi, A., Walsh, T.J., 2002. Pathogenic Aspergilus species recovered from a hospital water system: A 3-year prospective study. Clin. Infect. Dis., 34, 780-789.
Bennett, J.W., Klich, M., 2003. Mycotoxins. Clin. Microb. Rev., 16, 497-516.
Brown, B.Y., Moran, K., Brown, P.C., Reeves, E.S.G., 2004. The mold issue. Water instruction and toxic mold claims in Virginia Richmond, Virginia.
Cheesbrough, M., 2010. District laboratory practice in tropical Countries. Second edition update part 2. Cambridge University Press. 5 ISBN; 9780521676335, 1-339.
Cheesebrough, M., 2000. District laboratory practice in tropical countries. Part 2 Cambridge low price editions, 245-246 and 3-8.
De Hoog, G.S., Guarro, J., Gene, J., Figueras, M.J., 2005. Atlas of clinical fungi. Central bureay voor schimme clultures, Utrecht, the Netherlands.
De Villiers, M., Helway, G., 2002. Water and sustainability in the sub-saharan Africa. Can. J. Pol. Res., 3, 51-56.
Galgiani, J.N., Rex, J.H., Peller, N.H., 1997. Water treatment and pathogen control: Process efficiency in achieving safe drinking water. Edited by Mark W LeChevallier and Kwok-Keung Au. ISBN: 1 84339 069 8. Published by IWA Publishing, London, UK.
Gunhild, H., Nelson, L., Ida, S., 2009. The study of fungi in drinking water. Microbiol. Res., 113, 165-172.
Kanzler, D., Buzina, W., Paulitsch, A., Haas, D., Platzer, S., Marth, E., Mascher, F., 2007. Occurrence and hygienic relevance of fungi in drinking water. J. Compil., 51(2), 165-169.
Morgan, J., Wannemuchler, K.A., Marr, K.A., Hadley, S., Kontoyiannis, D.P., Walsh, T.J., Fridkin, S.K., Pappas, P.G., Warnock, D.W., 2005. Incidence of invasive asperfillosis following haematopietic stem cell and solid organ transportation: interim result of prospective multicenter surveillance programme. Med. Mycol., 43(suppl.1), 549-558.
Okpako, A.N., Osuagwu, A.E., Duke, N., 2009. Prevalence and borehole drinking water in Calabar, Nigeria. Afr. J. Microb. Res., 3(2), 056-061.
Paterson, R.R.M., Lima, N., 2005. Fungal contamination of drinking water. In water encyclopedia; Lehr, J., Keeley, J., Lehr, J., Kingeny III, T.B. (eds.) John whiley and sons.
Rogers, T.R., 2006. Antifungal drug resistance: limited data, dramatic impact. Int. J. Antimicrob. Agents, 1, 7-11.
World Health Organization, 2008. Guidelines for drinking water quality [electronic resources]: Incorporating 1st and 2nd addenda, Vol. 1, Recommendations -3rd edition.
Published
How to Cite
Issue
Section
Copyright (c) 2021 A.A. Usman, A.B. Zainab, A.P. Odeh, C.E. Ezeamagu

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.



