Phenotypic and genotypic characterization of a marine bacterium isolated from Sundarbans, Bangladesh
Keywords:
Phenotypic, Genotypic, Marine Bacteria, SundarbansAbstract
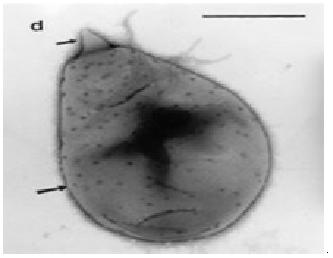
The mangrove ecosystem is a largely unexplored source for bacteria with potentiality to produce biologically active secondary metabolites. Consequently, we set our research study to isolate, characterize and screen a bacterium producing bioactive compounds from marine soil of largest mangrove forest of the world, Sundarbans, Bangladesh. A total number of 39 marine bacterial colonies were isolated and purified. Representative bioactive isolate, ANAM-5 was characterized using phenotypic and genotypic procedures since it exhihibited highest antibacterial activity against a series of test organisms. The 16S rDNA sequence of the strain ANAM-5 showed close similarity with only one bacterium Planctomyces brasiliensis, complete genome (99.64%). But the cultural, morphological, physiological and biochemical characteristics of the strain and P. brasiliensis were completely different. So, it may not be logical to assign the strain ANAM-5 to the genus Planctomyces. Thus, to confirm the taxonomic position of the strain and to have a correct solution from this taxonomic problem, further studies is needed in respect to the DNA relatedness studies, fatty acid profiling, DNA-DNA hybridization, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), metabolic finger profiling using BIOLOG, ribotyping, small subunit (SSU) sequences, cell wall composition and other characterizations.
References
Adekambi, T., Colson, P., Drancourt, M., 2003. RpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 41, 5699-5708.
Alcamo, E., Pomerville, J.C., 2004. Alcamo’s laboratory fundamental of microbiology.
Anderson, A.S., Wellington, E.M.H., 2001. The taxonomy of Streptomyces and related genera. Int. J. Syst. Evol. Microbiol. 51, 797–814.
Bernard, B., 2007. Access excellence @ the national health museum, isolation of antibiotic strains from soils. (www. Access excellence. Org.).
Bull, A.T., 2004. Microbial diversity and biosprospecting. ASM Press.
Christopher, K., Bruno, E., 2003. Identification of bacterial species. In tested studies for laboratory teaching. Ed., O’Donnell MA. Proceedings of the 24th Workshop/Conf. Assoc. Biol. Lab. Educ. 24, 103-130.
Clarridge, J.E., 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17(4), 840-862.
Cloud, J.L., Hoggan, K., Belousov, E., Cohen, S., Brown-Elliott, B.A., Mann, L., Wilson, R., Aldous, W., Wallace, R.J., Jr Woods, G.L., 2005. Use of the MGB eclipse system and smart cycler PCR for differentiation of Mycobacterium chelonae and M. abscessus. J. Clin. Microbiol. 43, 4205–4207.
Colwell, R.R., 1970. Polyphasic taxonomy of the genus Vibrio: numerical taxonomyof Vibrio cholerae, Vibrio parahaemolyticus, and related Vibrio species. J. Bacteriol. 104, 410–33.
Drancourt, M., Bollet, C., Carlioz, A., Martelin, R., Gayral, J.P., Raoult, D., 2000. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbi. 38, 3623-3630.
Eardly, B.D., Wang, F.S., Berkum, P.V., 1996. Corresponding 16S rRNA gene segments in Rhizobiaceae and Aeromonas yield discordant phylogenies. Plant. Soil. 186, 69–74.
Farrand, S.K., Berkum, P.V., Ger, P., 2003. Agrobacterium is a definable genus of the family Rhizobiaceae. Int. J. Syst. Evol. Microbiol. 53, 1681–7.
Fox, G.E., Wisotzkey, J.D., Jurtshuk, P., Jr, 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42, 166-170.
Fuerst, J. A., Gwilliam, H.G., Lindsay, M., Lichanska, A.,, Belcher, C., Vickers, J.E., Hugenholtz, P., 1997. Isolation and molecular identification of planctomycete bacteria from postlarvae of the giant tiger prawn, Penaeus monodon. App. Environ. Microbiol. 63(1), 254-262.
Gevers, D., Cohan, F.M., Lawrence, J.G., 2005. Re-evaluating prokaryotic species. Nat. Rev. Microbiol. 3, 733–9.
Ghosh, A., Bhardwaj, M., Satyanarayana,T., Khurana, M., Mayilraj, S., Jain, R.K., 2007. Bacillus lehensis sp. nov. an alkali tolerant bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 57, 238–42.
Goris, J., Konstantinidis, K.T., Klappenbach, J.A., 2007. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57, 81-91.
Gupta, R., Lanter, J.M., Woese, C.R., 1983. Sequence of the 16S ribosomal RNA from Halobacterium volcanii, an Archaebacterium. Sci. 221, 656–659.
Hobes, G., Frazer, C.M., Gardner, D.C.J., Flett, F., Oliver, S.G., 1990. Pigmented antibiotic production by S. coelicolor A3(2), kinetics and the influence of nutrients. J. Gen. Microb. 136, 2291-2296.
Imada, C., Koseki, N., Kamata, M., Kobayashi, T., Hamada-Sato, N., 2007. Isolation and characterization of antibacterial substances produced by marine actinomycetes in the presence of seawater. Actinomyce. 21, 27-31.
Janda, J.M., Abbot, S.L., 2007. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J. Clin. Microbiol. 45(9), 2761-2764.
Jonsbu, E.M., Intyre, M., Nielsen, J., 2002. The influence of carbon source and morphology on nystatin production by Streptomyces noursei. J. Biotec. 95, 133-144.
Knowles, D.J.C., 1997. New strategies for antibacterial drug design. Tre. Micro. 5(10), 379-383.
Korzybski, T., Kowszyk, G., Kurilowicz, W., 1967. Antibiotics, origin, nature and properties, translated by parayski. E. Per Pre, Oxf. 47, 450-725.
Kwok, A.Y., Su, S.C., Reynolds, R.P., 1999. Species identification and phylogenetic relationships based on partial HSP60 gene sequences within the genus Staphylococcus. Int. J. Syst. Bact. 49(3), 1181–1192.
Kwok, A.Y., Chow, A.W., 2003. Phylogenetic study of Staphylococcus and Macrococcus species based on partial hsp60 gene sequences. Int. J. Syst. Evol. Microb. 53, 87–92.
Lau, S.K., Woo, P.C., Woo, G.K., Yuen, K.Y., 2002. Catheterrelated Microbacterium bacteremia identified by 16S rRNA gene sequencing. J. Clin. Microbiol. 40, 2681–2685.
Levy, S.B., 1998. The challenge of antibiotic resistance. Sci. Am. 278(3), 32-39.
Mansour, F.A., El-Shirbiny, S.A., El- Metwaly, N.A., 1996. Demethyl tetracycline biosynthesis by Streptomyces aureofaciens Sub-species viridulans as influenced by medium composition. Egyp. J. Microbiol. 31, 221-235.
Miller, S.R., Augustine, S., Olson, T.L., Blankenship, R.E., Selker, J., 2005. Discovery of a free-living chlorophyll producing cyanobacterium with a hybrid proteobacterial/cyanobacterial small-subunit rRNA gene. Proc. Nat. Acad. Sci. USA. 102, 850-855.
Neidleman, S.L., Laskin, A.L., 2000. Adv. AppL. Micro. ElsE. 47, 88-90.
Palys, T., Nakamura, L.K., Cohan, F.M., 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Evol. Microbiol. 47, 1145–56.
Petti, C.A., 2007. Detection and identification of microorganisms by gene amplification and sequencing. Clin. Infect. Dis. 44, 1108–1114.
Phumudzo, T., Nnzeru, R., Ntushelo, K., Mudau, F., 2013. Bacterial species identification getting easier. Afr. J. Biotech. 12(41), 5975-5982.
Rajendhran, J., Gunasekaran, P., 2011. Microbial phylogeny and diversity: Small subunit ribosomal RNA sequence analysis and beyond. Microbiol. Res. 166, 99-110.
Ravikumar, S., Inbaneson, S.J., Uthiraselvam, M., Priya, S.R., Ramu, A., Banerjee, M.B., 2011. Diversity of endophytic actinomycetes from Karangkadu mangrove ecosystem and its antibacterial potential against bacterial pathogens. J. Pharm. Res. 4(1), 294-296.
Scheuner, C., Tindall, B.J., Lu, M., Nolan, M., Lapidus, A., Cheng, J.F., Goodwin, L., Pitluck, S., Huntemann, M., Liolios, K., Pagani, I., Mavromatis, K., Hauser, L., Land, M., Mwirichia, R., Rohde, M., Abt, B., Detter, J.C., Woyke, T., Eisen, J.A., Markowitz, V., Hugenholtz, P., Mgöker, M., Kyrpides, N.C., Klenk, H.P., 2014. Complete genome sequence of Planctomyces brasiliensis type strain (DSM 5305T), phylogenomic analysis and reclassification of Planctomycetes including the descriptions of Gimesia gen. nov., Planctopirus gen. nov. and Rubinisphaera gen. nov. and emended descriptions of the order Planctomycetales and the family Planctomycetaceae. Stand. Gen. Sci. 9(10), 356-372.
Schlesner, H., 1989. Planctomyces brasiliensis sp. Nov., a halotolerant bacterium from a salt pit. Syst. Appl. Microbiol. 12, 159–61.
Schouls, L.M., Schot, C.S., Jacobs, J.A., 2003. Horizontal transfer of segments of the 16S rRNA genes between species of the Streptococcus anginosus group. J. Bacteriol. 185, 7241–6.
Shirling, E.B., Gottlieb, D., 1966. Methods of characterization of Streptomyces species. Int. J. Sys. Bactriol. 16(3), 313-340.
Solanki, R., Khanna, M., Lal, R., 2008. Bioactive compounds from marine actinomycetes. Ind. J. Microbiol. 48, 410–431.
Song, Y., Liu, C., McTeague, M., Finegold, S.M., 2003. 16S ribosomal DNA sequence-based analysis of clinically significant gram-positive anaerobic cocci. J. Clin. Microbiol. 41(4), 1363-1369.
Valli, S., Sugasini, S.S., Aysha, O.S., Nirmala, P., Kumar, P.V., Reena, A., 2012. Antimicrobial potential of Actinomycetes species isolated from marine environment. Asi. Pac. J. Trop. Biom. 45, 469-474.
Vandamme, P., Pot, B., Gillis, M., de Vos, P., Kersters, K., Swings, J., 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60, 407–38.
Wang, Y., Zhang, Z., Ramanan, N., 1997. The actinomycete Thermobispora bispora contains two distinct types of transcriptionally active16S rRNA genes. J. Bacteriol. 179, 3270–6.
Wayne, L.G., Brenner, D.J., Colwell, R.R., 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37, 463–4.
Williams, S.T., Goodfellow, M., Alderson, G., Wellington, E.M.H., Sneath, P.H., Sackin, M., 1983. Numerical classification of Streptomyces and related genera. J. Gen. Appl. Microbiol. 129, 1743–1813.
Woo, P.C., Chong, K.T., Leung, K., Que, T., Yuen, K., 2001. Identification of Arcobacter cryaerophilus isolated from a traffic accident victim with bacteremia by 16S ribosomal RNA gene sequencing. Diag. Microbi. Infect. Dis. 40, 125–127.
Woo, P.C., Leung, A.S., Leung, K.W., Yuen, K.Y., 2001. Identification of slide-coagulase positive, tube-coagulase negative Staphylococcus aureus by 16S ribosomal RNA gene sequencing. Mol. Pathol. 54, 244–247.
Woo, P.C., Ngan, A.H., Lau, S.K., Yuen, K.Y., 2003. Tsukamurella conjunctivitis: a novel clinical syndrome. J. Clin. Microbiol. 41, 3368–3371.
Woo, P.C.Y., Teng, J.L.L., Wu, J.K.L., Leung, F.P.S., Tse, H., Fung, A.M.Y., Lau, S.K.P., Yuen, K.Y., 2009. Guidelines for interpretation of 16S rRNA gene sequence-based results for identification of medically important aerobic Gram-positive bacteria. J. Medi. Microbiol. 58, 1030–1036.
Young, J.M., Kuykendall, L.D., Martinez-Romero, E., Kerr, A., Sawada, H., 2001. A revision of Rhizobium Frank 1889, with anemended description of the genus, and the inclusion of all species of Agrobacterium Conn, 1942 and Allorhizobium undicola de Lajudie etal., 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 51, 89–103.
Zhao, H., Parry, R.L., Fllis, D.L., Griffith, G.W., Goodacre, R., 2006. The rapid differentiation of Streptomyces isolates using fourier transform infrared spectroscopy. Vibrat. Spec. 40, 213-218.
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Ashish Kumar Sarker, Anwarul Haque, Mohammad Sayful Islam, Uzzal Haque, Netish Kumar Kundo, Abu Syed Anisuzzaman, Urmi Saha, Anwar Ul Islam

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.



